Trending...
- Where You Play Matters: MrBet Decodes Europe's Complex Gambling Tax Rules in New Report
- Who the Hell is Cloud9 Network—And Why Is Everyone Suddenly Watching?
- A Quality 4.0 roadmap is now available!
MUNICH - ncarol.com -- OncoBeta® GmbH, a medical device company specialising in innovative epidermal radioisotope therapies, has announced 12-months interim results from its international Phase IV, multi-centre clinical trial evaluating the efficacy and safety of Rhenium-SCT® (Skin Cancer Therapy) in patients with non-melanoma skin cancer (NMSC).
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT for the Treatment of Non-Melanoma Skin Cancer) is designed to assess treatment efficacy and safety, as well as key patient-reported outcomes including quality of life, treatment comfort, and cosmetic results.1 The study is based on the clinically validated effect of the beta-emitter rhenium-188 in treating basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2
Treatment data is available for 184 adult patients (with 254 lesions) across 7 study centres in Australia, Austria, Germany, the United Kingdom, and South Africa. All participants had histologically confirmed stage I or II NMSC. The median patient age was 70.3 years (range 27–95 years), and 53.3% of participants were male. Patients had one (71.2%), two (19.6%), or three (9.2%) lesions treated, which were located on the head & neck (11.8%), trunk (11.8%), lower limbs (11.8%), or upper limbs (9.1%).1
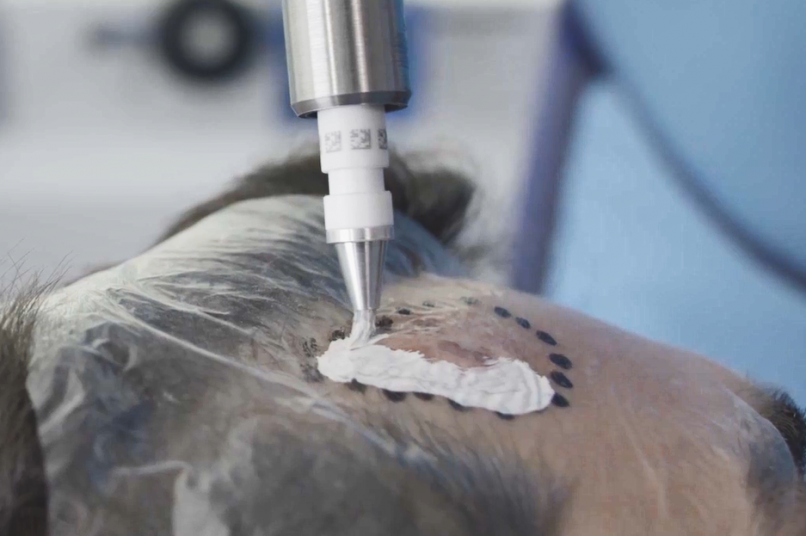
Treatment & Methodology
Rhenium-SCT was administered as a single 50-Gy topical dose of rhenium-188 embedded in a resin applied via adhesive film to the lesion site. Efficacy was assessed using modified RECIST criteria, at 12 months follow-up. Quality of life was assessed using the Skin Cancer Index (SCI) at baseline, 6 months, and 12 months. Treatment comfort was assessed using a patient questionnaire, whilst patient- and clinician-evaluated cosmetic outcomes were determined using a visual analogue scale (VAS) grading of 0-10 (0 = very poor; 10 = no wound detectable). Safety was assessed via treatment-emergent adverse events (TEAEs) and CTCAE (Common Terminology Criteria for Adverse Events) grading.
Key 12-Month Results:1
These findings confirm that Rhenium-SCT®, administered in a single session, delivers sustained high efficacy and improved quality of life, with excellent cosmetic outcomes and minimal adverse effects.
More on ncarol.com
"Rhenium-SCT® has consistently demonstrated effectiveness and safety in prior studies," said Dr. Gerhard Dahlhoff, Medical Director at OncoBeta®. "This 12-month interim data further reinforces its value as a non-invasive, targeted treatment option for NMSC, particularly for patients seeking alternatives to surgery due to cosmetic or health-related concerns."
"The 12-month results from the EPIC-Skin Study represent a major milestone for OncoBeta®," added Shannon D. Brown III, CCO OncoBeta & Managing Director Europe. "These results support the efficacy, safety, and patient satisfaction associated with Rhenium-SCT®. These are the three foundational pillars of our patient-first vision, emphasising not only clinical outcomes, but also the patient experience in achieving them."
_
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.3 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.4
The Rhenium-SCT is a painless*, non-invasive‡ therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,5,6 The Rhenium-SCT utilises the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy2 that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6-8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta® has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers and keloid scars. OncoBeta® has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
More on ncarol.com
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties, and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No pain reported during procedure.6
‡No direct skin contact or incisions.6
References
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT for the Treatment of Non-Melanoma Skin Cancer) is designed to assess treatment efficacy and safety, as well as key patient-reported outcomes including quality of life, treatment comfort, and cosmetic results.1 The study is based on the clinically validated effect of the beta-emitter rhenium-188 in treating basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2
Treatment data is available for 184 adult patients (with 254 lesions) across 7 study centres in Australia, Austria, Germany, the United Kingdom, and South Africa. All participants had histologically confirmed stage I or II NMSC. The median patient age was 70.3 years (range 27–95 years), and 53.3% of participants were male. Patients had one (71.2%), two (19.6%), or three (9.2%) lesions treated, which were located on the head & neck (11.8%), trunk (11.8%), lower limbs (11.8%), or upper limbs (9.1%).1
Treatment & Methodology
Rhenium-SCT was administered as a single 50-Gy topical dose of rhenium-188 embedded in a resin applied via adhesive film to the lesion site. Efficacy was assessed using modified RECIST criteria, at 12 months follow-up. Quality of life was assessed using the Skin Cancer Index (SCI) at baseline, 6 months, and 12 months. Treatment comfort was assessed using a patient questionnaire, whilst patient- and clinician-evaluated cosmetic outcomes were determined using a visual analogue scale (VAS) grading of 0-10 (0 = very poor; 10 = no wound detectable). Safety was assessed via treatment-emergent adverse events (TEAEs) and CTCAE (Common Terminology Criteria for Adverse Events) grading.
Key 12-Month Results:1
- Overall response rate: 97.3%
- Complete response rate: 94.1%
- Partial response rate: 3.2%
- Mean improvement in quality of life: +10.55 points from baseline
- Pain/discomfort during treatment: None reported
- Cosmetic outcomes: Favourable results reported by both patients and clinicians
- Most common toxicity (12-month): Grade 1 hypopigmentation (60.4%)
- No CTCAE toxicities above Grade 2 observed at 12 months
These findings confirm that Rhenium-SCT®, administered in a single session, delivers sustained high efficacy and improved quality of life, with excellent cosmetic outcomes and minimal adverse effects.
More on ncarol.com
- Hundreds Attend Annual Purple Heart Day Banquet Hosted by the Citizens Commission on Human Rights at the Historic Fort Harrison
- 3,000+ Business Owners Create Tasks in One Click and Plan Their Day in Seconds: Voiset App Gets Major Speed Upgrade
- $100 Million Raise Initiative Launched via Share Offering at $4 Level for Cryptocurrency and Real Estate Development Project Company: OFA Group $OFAL
- Author Lorilyn Roberts Explores Humanity's Origins in New YA Sci-Fi Thriller, The Eighth Dimension: Frequency
- Dr. Samuel Waymon to represent the USA and the legacy of Nina Simone in Barbados at CARIFESTA XV
"Rhenium-SCT® has consistently demonstrated effectiveness and safety in prior studies," said Dr. Gerhard Dahlhoff, Medical Director at OncoBeta®. "This 12-month interim data further reinforces its value as a non-invasive, targeted treatment option for NMSC, particularly for patients seeking alternatives to surgery due to cosmetic or health-related concerns."
"The 12-month results from the EPIC-Skin Study represent a major milestone for OncoBeta®," added Shannon D. Brown III, CCO OncoBeta & Managing Director Europe. "These results support the efficacy, safety, and patient satisfaction associated with Rhenium-SCT®. These are the three foundational pillars of our patient-first vision, emphasising not only clinical outcomes, but also the patient experience in achieving them."
_
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.3 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.4
The Rhenium-SCT is a painless*, non-invasive‡ therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,5,6 The Rhenium-SCT utilises the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy2 that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6-8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta® has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers and keloid scars. OncoBeta® has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
More on ncarol.com
- This Sunday on "Financial Freedom with Tom Hegna" Larry and Judy Poole
- Larry & Judy Poole Join Tom Hegna on "Financial Freedom with Tom Hegna"
- EZsolutions Launches EZ AI with Proprietary AI Test Matrix to Revolutionize AI Search Optimization for Local Businesses
- Dr. Mark Dill Expands Dental Technology Suite in Cleveland, TN with 3D Printing and Panoramic Imaging
- Assent's New EU Deforestation Regulation Solution Helps Manufacturers Ensure Readiness for Urgent Deadline
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties, and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No pain reported during procedure.6
‡No direct skin contact or incisions.6
References
- Cardaci G, et al. Adv Radiat Oncol. 2025;10(7):101802.
- Cipriani C, et al. J Dermatol Treat. 2022;33(2):969–975. Epub 22 Jul 2020.
- Ciążyńska M, et al. Sci Rep. 2021;11(1):4337.
- Cancer.net. Skin Cancer (Non-Melanoma): Risk Factors and Prevention. February 2022. https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/risk-factors-and-prevention (accessed June 2025).
- Tietze JK, et al. Clin Nuc Med. 2023;48(10):869–876.
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021;48(5):1511–1521.
- Cipriani C, et al. Int J Nucl Med. 2017;114–112.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745–749.
Source: OncoBeta GmbH
Filed Under: Health
0 Comments
Latest on ncarol.com
- Genpak Increases Foodservice Packaging Efficiency for Emerging Restaurants
- Where You Play Matters: MrBet Decodes Europe's Complex Gambling Tax Rules in New Report
- $1 Million Equity Dividend Ignites Investor Frenzy as AI & Cybersecurity Titans IQSTEL (N A S D A Q: IQST) & Cycurion (N A S D A Q: CYCU) Join Forces
- From Paris to Rome to Venice — The World's No.1 Superstar™ Claims Another City with Bad Boy's Wild Ride
- Digital Dawn Launches Revolutionary Bitcoin Copy Trading Platform With 30-40% Monthly Returns
- Letter Four Launches Design-Build Decoded™ Podcast: Demystifying Architecture and Construction with AI Hosts
- Magnificent 63-Acre Equestrian Estate in Maryland's Horse Country Lists for $5.75M
- Shaping the Future of Congresses and Conventions: Osvaldo Rivera's Double Win at LAWA 2025
- Louisiana's First Dedicated Residential Eating Disorder Treatment Center for Women Opens Near New Orleans
- Post-Traditional Career Expert Sandra Buatti-Ramos Receives 2025 Top Career Coach Recognition
- CCHR Calls for Audit of Forced Psychiatric Drugging and Systemic Misdiagnosis
- St. Paul, Mn Goes Ninja: Emmy-winning Naruto Star Maile Flanagan Headlines Inaugural Anime Wonder Fest
- Local Author Heather Barbour Fenty Releases Third Book in Beloved "Sea Series"
- FDA Fast Track Designation for $3 Billion Suicidal Depression Market May Soon be Accessible for Effective NRX 100 Drug Therapy: NRx Pharmaceuticals
- Slotozilla Expands Partner Network With 25+ Affiliates and Unveils 139 New Bonuses in Q2 2025
- Who the Hell is Cloud9 Network—And Why Is Everyone Suddenly Watching?
- Newport Heights' Most Coveted Coastal Estate
- A Quality 4.0 roadmap is now available!
- Bay Miner Unveils Crypto Mining App to Help Investors Seize 2025's XRP Compliance Investment Opportunities
- Arms Preservation Inc Offers Industry-Leading VCI Firearm Storage Bags for Superior Rust Prevention