Trending...
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- Caraline Skincare's Gentle Glow Cleansing Oil Named Finalist for Best Face Cleanser at the 2026 CertClean Clean Beauty Awards
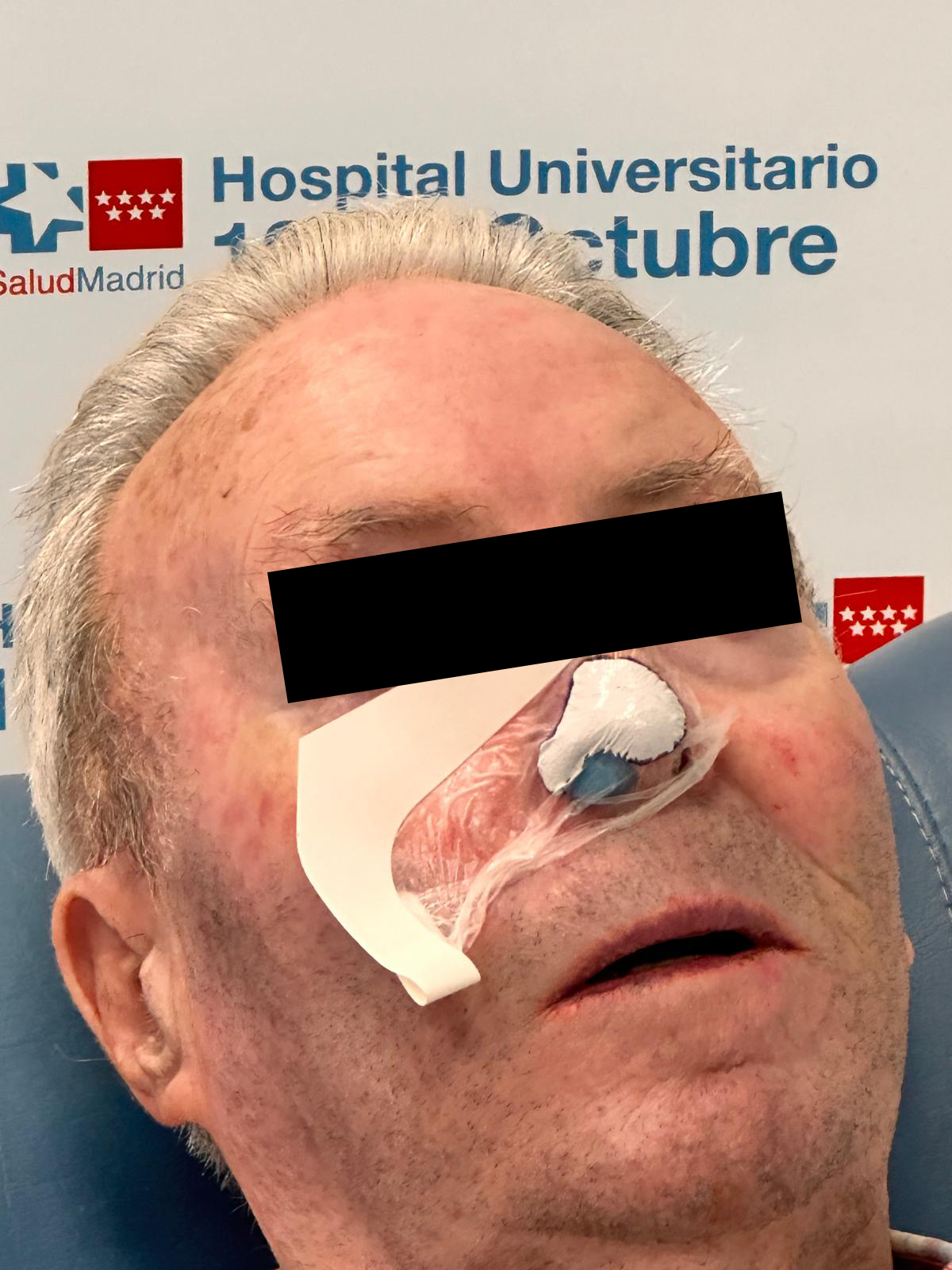
MADRID - ncarol.com -- OncoBeta®, a leading medical device company specialising in innovative epidermal radioisotope therapies, today announced Hospital Universitario 12 de Octubre, in Madrid, has treated its first patient with Rhenium-SCT® (skin cancer therapy) – and is expected to continue offering this therapy for patients with non-melanoma skin cancers (NMSCs).
Rhenium skin cancer therapy is an advanced radionuclide therapy technology that offers a painless,*1,2 single-session,†1-3 non-invasive‡1 treatment without disfiguring scarring.1,4 Treatment with Rhenium-SCT employs a superficial application of a paste containing β-emitting particles directly to the NMSC lesion, targeting cancer cells without the need for surgery.1,4
Shannon D. Brown III, CCO at OncoBeta, says, "With rates of NMSC increasing globally, we feel it's vitally important that new treatment options are offered to patients. Surgical intervention continues to be the most common treatment, but it can lead to disappointing results. Rhenium skin cancer therapy addresses many patients' desires for a non-surgical alternative – with simple application directly to the lesion, treatment time generally ranges 45 to 180 mins, which means patients can continue with their lives with very little disruption."
New cases of NMSCs in Spain are expected to almost double from 2022-2050 according to latest figures by the International Agency of Research on Cancer as part of the World Health Organization,5 making Mardrid an important location for this innovative NMSC treatment option.
"Rhenium-SCT has long been accessible to patients across Europe. This new clinic in Spain marks a significant milestone in expanding access to this innovative treatment for non-melanoma skin cancer patients," Mr Brown explains.
Dr Gerhard Dahlhoff, Medical Director at OncoBeta, says, "Rhenium-SCT allows for a targeted and non-invasive treatment of NMSCs without damaging adjacent healthy tissue. It offers a patient-friendly treatment alternative and we are delighted to have another medical facility offering Rhenium-SCT to those suffering with NMSCs".
More on ncarol.com
___
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.1 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.1,4
The Rhenium-SCT is a painless,*1,2 single session,†1-3 non-invasive‡1 therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,4,6 The Rhenium-SCT utilizes the radioisotope rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalized therapy that is only applied to the area needed to treat without affecting the healthy tissue.4,6 The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (basal cell carcinomas and squamous cell carcinomas) can be treated using the Rhenium-SCT in one single session. †1-4 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.1,6,7
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
Find out more about the Rhenium-SCT at www.oncobeta.com
More on ncarol.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain during procedure.1,2
†Complete tumour regression in 98.5% of lesions treated.3
‡A treatment is considered non-invasive when no cut or break in the skin is created.8
References
Rhenium skin cancer therapy is an advanced radionuclide therapy technology that offers a painless,*1,2 single-session,†1-3 non-invasive‡1 treatment without disfiguring scarring.1,4 Treatment with Rhenium-SCT employs a superficial application of a paste containing β-emitting particles directly to the NMSC lesion, targeting cancer cells without the need for surgery.1,4
Shannon D. Brown III, CCO at OncoBeta, says, "With rates of NMSC increasing globally, we feel it's vitally important that new treatment options are offered to patients. Surgical intervention continues to be the most common treatment, but it can lead to disappointing results. Rhenium skin cancer therapy addresses many patients' desires for a non-surgical alternative – with simple application directly to the lesion, treatment time generally ranges 45 to 180 mins, which means patients can continue with their lives with very little disruption."
New cases of NMSCs in Spain are expected to almost double from 2022-2050 according to latest figures by the International Agency of Research on Cancer as part of the World Health Organization,5 making Mardrid an important location for this innovative NMSC treatment option.
"Rhenium-SCT has long been accessible to patients across Europe. This new clinic in Spain marks a significant milestone in expanding access to this innovative treatment for non-melanoma skin cancer patients," Mr Brown explains.
Dr Gerhard Dahlhoff, Medical Director at OncoBeta, says, "Rhenium-SCT allows for a targeted and non-invasive treatment of NMSCs without damaging adjacent healthy tissue. It offers a patient-friendly treatment alternative and we are delighted to have another medical facility offering Rhenium-SCT to those suffering with NMSCs".
More on ncarol.com
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- STEM Agent Academy Mission 01: Reclaiming Children's Curiosity from the Digital Void
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
___
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.1 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.1,4
The Rhenium-SCT is a painless,*1,2 single session,†1-3 non-invasive‡1 therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,4,6 The Rhenium-SCT utilizes the radioisotope rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalized therapy that is only applied to the area needed to treat without affecting the healthy tissue.4,6 The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (basal cell carcinomas and squamous cell carcinomas) can be treated using the Rhenium-SCT in one single session. †1-4 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.1,6,7
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
Find out more about the Rhenium-SCT at www.oncobeta.com
More on ncarol.com
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain during procedure.1,2
†Complete tumour regression in 98.5% of lesions treated.3
‡A treatment is considered non-invasive when no cut or break in the skin is created.8
References
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021; 48: 1511–1521.
- Cipriani C, et al. J Dermatol Treat. 2022;33(2):969–975. Epub 22 Jul 2020.
- Cipriani C, et al. In Therapeutic Nuclear Medicine. 2014. RP Baum (Ed), New York: Springer.
- Tietze JK, et al. Clin Nucl Med. 2023;48:869–76.
- Global Burden of Disease Cancer Collaboration, et al. JAMA Oncol. 2019;5(12):1749-1768.
- Cipriani C, et al. International J Nucl Med. 2017; July:114–112.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745–749.
- Australian Therapeutic Goods Administration. Acronym and glossary of terms. Available at: https://www.tga.gov.au/resources/acronyms-and-glossary-terms. (Accessed June 2024).
Source: OncoBeta GmbH
0 Comments
Latest on ncarol.com
- $38 Million in U.S. Government Contract Awards Secured Through Strategic Partner. Establishing Multi-Year Defense Revenue Platform Through 2032: $BLIS
- Mecpow M1: A Safe & Affordable Laser Engraver Built for Home DIY Beginners
- CrashStory.com Launches First Colorado Crash Data Platform Built for Victims, Not Lawyers
- Inkdnylon Earns BBB Accreditation for Verified Business Integrity
- Josh Stout "The Western Project"
- Open House Momentum Builds at Heritage at South Brunswick
- A Celebration of Visibility, Voice and Excellence: The 57th NAACP Image Awards Golf Invitational, Presented by Wells Fargo, A PGD Global Production
- Athens in Spring: A Culinary City Break That Rivals Paris and Copenhagen
- ClearSight Therapeutics Signs LOI with Covalent Medical for $60M Multi-Channel OTC Eye Care Partnership
- Jayne Williams Joins Century Fasteners Corp. Sales and Business Development Team
- Rocket Fibre Services Growing Customer Base With netElastic Networking Software
- Cummings Graduate Institute for Behavioral Health Studies Honors New Doctor of Behavioral Health Graduates
- IDpack v4 Launches: A Major Evolution in Cloud-Based ID Card Issuance
- CCHR Says Psychiatry's Admission on Antidepressant Withdrawal Comes Far Too Late
- 505 Plumbing, Heating & Cooling Launches in Albuquerque, Bringing a Customer-First Approach to Home Services
- As AI.com Sells For Record $70 Million, Attention Now Turns To ArtificialIntelligence.com
- AOW Event Sponsored By The Stanglwirt Resort a renowned five-star Austrian wellness destination
- Average US gambler spends $210 per month in 2026
- 10X Recruitment Launches Operator-Led Executive Search for Behavioral Health and Legal Leaders
- Integris Composites developing armor for military in Arctic Circle