Trending...
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
FAYETTEVILLE, Ark. - ncarol.com -- Lineus Medical is pleased to announce the appointment of Matt Stuckert to its Board of Directors. Stuckert was previously a member of the Lineus Technical Advisory Board. Stuckert brings over a decade of sales leadership experience in the vascular access space, and has held key roles at Magnolia Medical, Becton Dickinson, CareFusion, Cardinal Health, Enturia, and Venetec.. Throughout his career, he has successfully built and led sales teams, and has driven commercial growth, particularly in the adoption of novel new vascular technologies.
His expertise in sales strategy and business development will be instrumental in guiding Lineus Medical's continued success. With a deep understanding of the vascular access market, Stuckert's knowledge will help support the growth of the company's flagship product, SafeBreak Vascular. Stuckert commented, "I am excited to join Lineus Medical's Board of Directors and contribute to the company's growth. SafeBreak Vascular is a game-changing vascular access device that directly addresses IV complications and improves patient experience, both critical issues in hospitals. I look forward to leveraging my experience to help expand SafeBreak's adoption and support the company's mission of improving IV care."
More on ncarol.com
Vance Clement, CEO of Lineus Medical said, "Matt Stuckert's extensive experience in vascular access sales and marketing makes him a valuable addition to our Board. His insights will be instrumental as we expand SafeBreak Vascular's presence in hospitals nationwide. We are excited to have him join our board as we continue to revolutionize what vascular access care looks like."
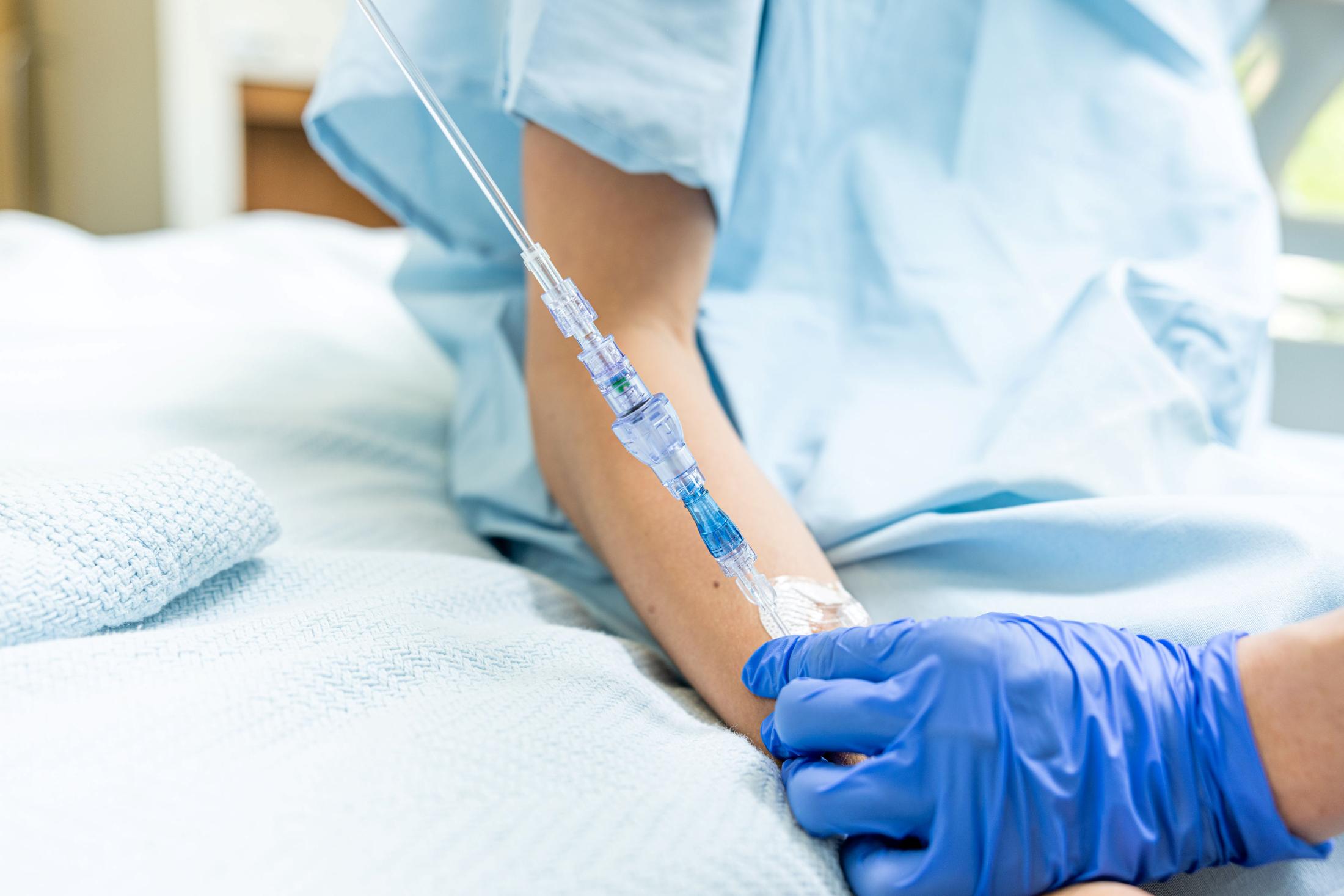
SafeBreak Vascular is the only break-away device for IV lines clinically proven to reduce IV complications¹. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close, preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money.1
About Lineus Medical
Lineus Medical is the developer of break-away technology proven to reduce IV restarts and complications¹. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn.
References:
1. Data on File
His expertise in sales strategy and business development will be instrumental in guiding Lineus Medical's continued success. With a deep understanding of the vascular access market, Stuckert's knowledge will help support the growth of the company's flagship product, SafeBreak Vascular. Stuckert commented, "I am excited to join Lineus Medical's Board of Directors and contribute to the company's growth. SafeBreak Vascular is a game-changing vascular access device that directly addresses IV complications and improves patient experience, both critical issues in hospitals. I look forward to leveraging my experience to help expand SafeBreak's adoption and support the company's mission of improving IV care."
More on ncarol.com
- Tarrytown Expocare Pharmacy Announces Strategic Leadership Appointments to Accelerate Growth and Innovation
- New Environmental Thriller "The Star Thrower" Reimagines a Classic Lesson in Individual Impact
- Summit Appoints Javier Cabeza as Data, AI, and Analytics Practice Lead
- Interview: Why I Sponsored Carson Ware - The Full Story
- March Is Skiing's Smartest Buying Window
Vance Clement, CEO of Lineus Medical said, "Matt Stuckert's extensive experience in vascular access sales and marketing makes him a valuable addition to our Board. His insights will be instrumental as we expand SafeBreak Vascular's presence in hospitals nationwide. We are excited to have him join our board as we continue to revolutionize what vascular access care looks like."
SafeBreak Vascular is the only break-away device for IV lines clinically proven to reduce IV complications¹. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close, preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money.1
About Lineus Medical
Lineus Medical is the developer of break-away technology proven to reduce IV restarts and complications¹. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn.
References:
1. Data on File
Source: Lineus Medical
0 Comments
Latest on ncarol.com
- P-Wave Classics to publish Robert Bage's Hermsprong in three volumes, beginning 12 May
- Ease Auto Group Inc. & Ease Fleet Repair Service Celebrate Grand Opening Charlotte, North Carolina
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- Arcuri Group Announces Long‑Term Partnership with WakeMed Health & Hospitals to Deliver Situational Awareness and De‑escalation Training
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
- Juego Studios Extends Full-Cycle Game Development & Outsourcing Capabilities to the UAE Market
- VENUS Goes Live on CATEX Exchange As UK Financial Ltd Activates The Premier Division Of The Maya Meme's League
- Atlanta Tech Founder Seeks Clarity on Intellectual Property and Innovation Policy
- FPTower Inc. nonprofit launches coastal safety raffle fundraiser
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- STEM Agent Academy Mission 01: Reclaiming Children's Curiosity from the Digital Void
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract