Trending...
- Lick Personal Oils Introduces the Ultimate Valentine's Day Gift Collection for Romantic, Thoughtful Gifting
- Global License Exclusive Secured for Emesyl OTC Nausea Relief, Expanding Multi-Product Growth Strategy for Caring Brands, Inc. (N A S D A Q: CABR)
FAYETTEVILLE, Ark. - ncarol.com -- Lineus Medical is now officially registered in the United Kingdom, enabling the company to begin distributing SafeBreak® Vascular within the UK healthcare market. UK registration represents an important step in Lineus Medical's international expansion strategy, further extending access to SafeBreak Vascular for hospitals and clinicians on a global scale. With regulatory requirements in place, Lineus Medical can now work with distribution partners to bring its breakaway IV technology to the UK.
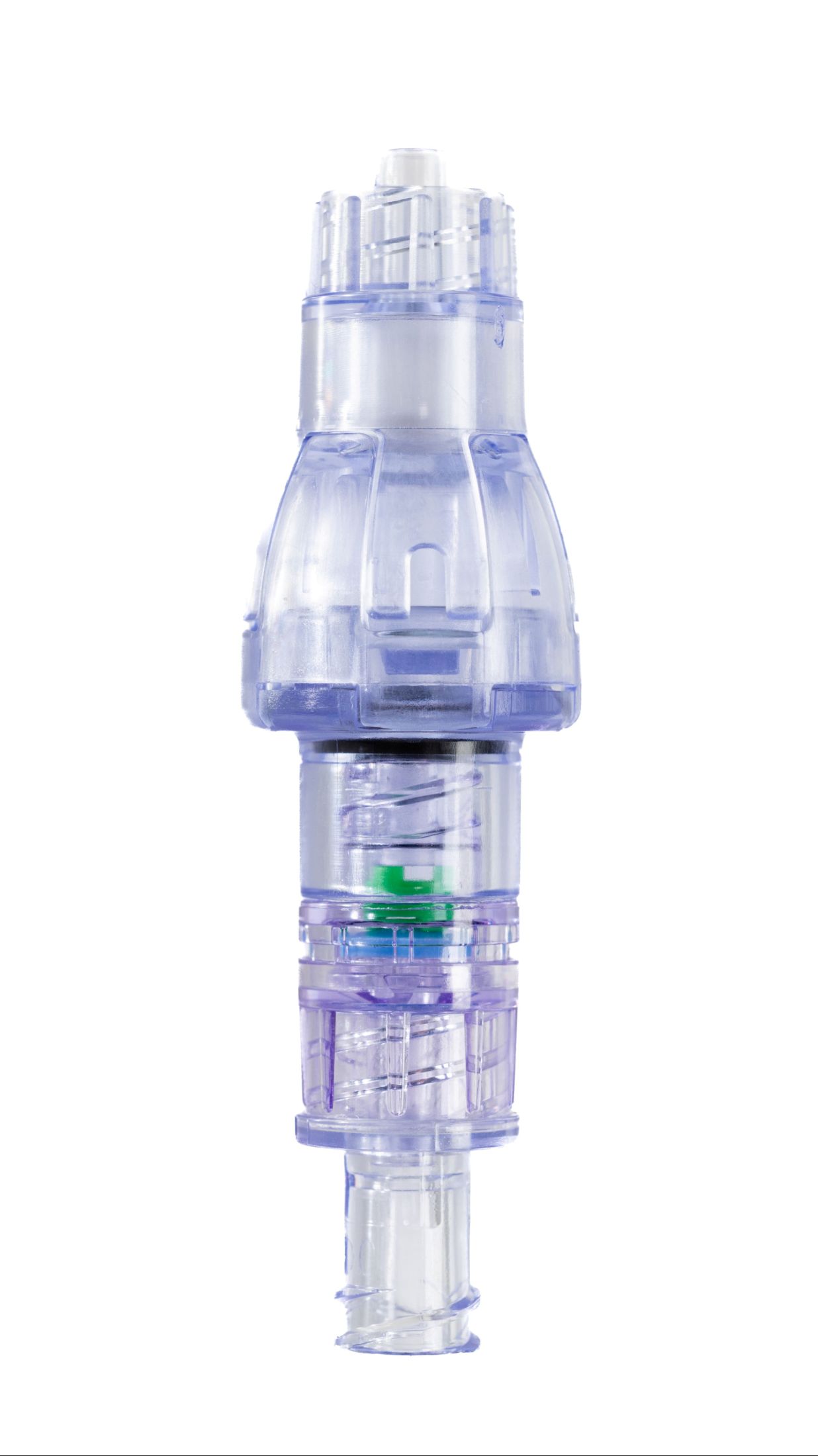
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on ncarol.com
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on ncarol.com
- Japan's Patented "Hammock'n" Smartphone Band Targets Hand Fatigue From Long Phone Use
- Reditus Group Introduces A New Empirical Model for Early-Stage B2B Growth
- CCHR: Harvard Review Exposes Institutional Corruption in Global Mental Health
- Goatimus Launches Dynamic Context: AI Prompt Engineering Gets Smarter
- Global License Exclusive Secured for Emesyl OTC Nausea Relief, Expanding Multi-Product Growth Strategy for Caring Brands, Inc. (N A S D A Q: CABR)
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
- Data on file.
Source: Lineus Medical
0 Comments
Latest on ncarol.com
- $140 to $145 Million in 2026 Projected and Profiled in New BD Deep Research Report on its Position in $57 Billion US Marine Industry; N Y S E: OTH
- Really Cool Music Releases Its Fourth Single - "So Many Lost Years"
- MGN Logistics Acquires Fast Service LLC, Fueling MyMGN Marketplace Expansion and Supercharging Expedited Coverage Nationwide
- The Wait is Over: Salida Wine Festival Announces Triumphant 2026 Return After Seven-Year Hiatus
- Graduates With $40K in Student Debt Are Buying Businesses Instead of Taking Entry-Level Jobs
- Tatt:Magic Unveils Breakthrough Tattoo Recovery System with Faster Recovery No Itch, for Vibrant ink
- Anne Seidman: Within the Lines
- How Democrats Made Healthcare More Expensive in 2026
- Inkdnylon Launches Bilingual Ask Inkdnylon Platform
- JS Gallery Brings Global Voices to LA Art Show 2026 with "OFF SCRIPT" Exhibition
- ANTOANETTA Partners With Zestacor Digital Marketing to Expand Online Presence for Handcrafted Luxury Jewelry
- FrostSkin Launches Kickstarter Campaign for Patent-Pending Instant-Chill Water Purification Bottle
- The New Monaco of the South (of Italy)
- Lick Personal Oils Introduces the Ultimate Valentine's Day Gift Collection for Romantic, Thoughtful Gifting
- Lacy Hendricks Earns Prestigious MPM® Designation from NARPM®
- Walmart $WMT and COSTCO.COM $COST Distribution as SonicShieldX™ Platform Sets the Stage for Accelerated Growth in 2026: AXIL Brands (N Y S E: AXIL)
- AI-Driven Drug Development with Publication of New Bioinformatics Whitepaper for BullFrog AI: $BFRG Strengthens Its Position in AI Drug Development
- IQSTEL Enters 2026 from a Position of Strength Following Transformational Year Marked by N A S D A Q Uplisting, Record Revenue and First-Ever
- Are You Hiring The Right Heater Repair Company in Philly?
- Charlotte Becomes the Epicenter of HBCU Culture: The 2026 BIG HBCU Southern Classic Returns