Trending...
- Gospel Sensation "Bible, Faith, Rembrandt aka BFR, They're Taking the World by Storm
- Building A Business Website That Works In 2025
- K-Drama Tours Expands Into K-Pop Experiences with New "K-Pop Demon Hunters" Tour in Seoul
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Suitability Petition is required for shift from multidose packaging of ketamine to single-patient dose preservative free ketamine
MIAMI - ncarol.com -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
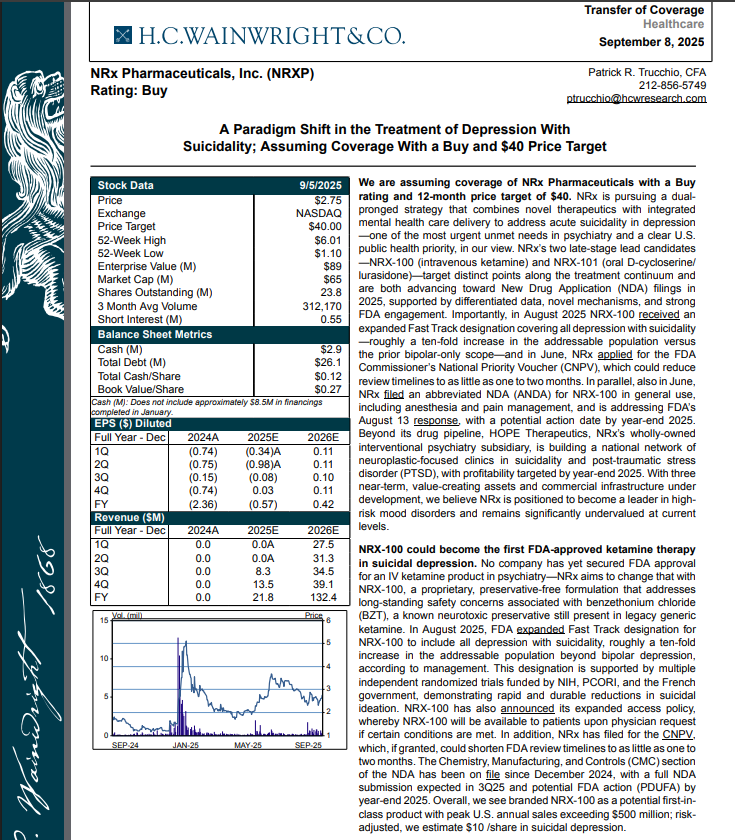
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
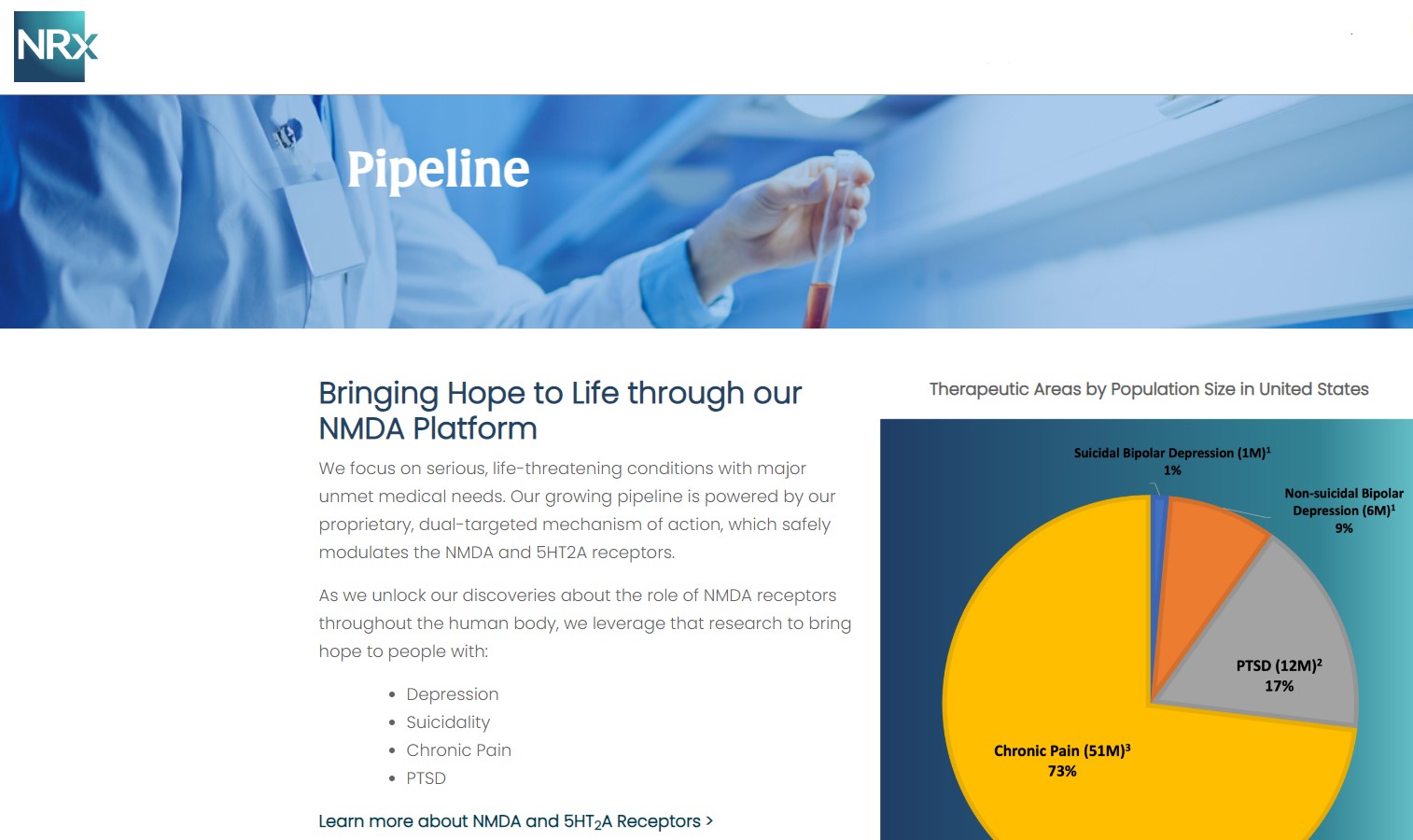
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on ncarol.com
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine
On September 24th NRXP announced that it was notified by the United States Food and Drug Administration (FDA) that a Suitability Petition has been granted for the strength proposed by the Company for its planned single-patient, preservative-free ketamine product (KETAFREE™).
Currently, ketamine is sold in multi-dose vials that contain Benzethonium Chloride, a toxic preservative. The Suitability Petition that has been granted enables immediate re-filing of the NRXP Abbreviated New Drug Application for KETAFREE™. NRXP believes that this proposed product addresses two critical policy objectives as articulated by the current administration: (1) the re-shoring of strategically important drugs, particularly sterile products from foreign manufacturing sources, and (2) the "Make America Healthy Again" (MAHA) objective of removing toxic preservatives and colorants from foods and drugs. These objectives have been articulated on numerous occasions by FDA and HHS leadership.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. NRXP believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
More on ncarol.com
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on ncarol.com
- Delirious Comedy Club Expands to Two Rooms and Secures Google's #1 Rated Comedy Club in Las Vegas
- SPOZZ, the Community-Owned Direct-to-Fan Music Ecosystem, adds "BEATS" — a Creator-to-Creator Marketplace
- DAECO Painting Sets the Gold Standard for High-End Interior Painting Services in Denver, CO
- Boston Industrial Solutions Expands Availability of Industry-Leading Primers to Mexico
- Unprescribed™ Introduces the Focus, Mood & Mind System™
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine
On September 24th NRXP announced that it was notified by the United States Food and Drug Administration (FDA) that a Suitability Petition has been granted for the strength proposed by the Company for its planned single-patient, preservative-free ketamine product (KETAFREE™).
Currently, ketamine is sold in multi-dose vials that contain Benzethonium Chloride, a toxic preservative. The Suitability Petition that has been granted enables immediate re-filing of the NRXP Abbreviated New Drug Application for KETAFREE™. NRXP believes that this proposed product addresses two critical policy objectives as articulated by the current administration: (1) the re-shoring of strategically important drugs, particularly sterile products from foreign manufacturing sources, and (2) the "Make America Healthy Again" (MAHA) objective of removing toxic preservatives and colorants from foods and drugs. These objectives have been articulated on numerous occasions by FDA and HHS leadership.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. NRXP believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
More on ncarol.com
- Texas Mechanic Unveils "Mighty Mule" Experimental Pontiac Engine—Delivering Over Triple the Factory Horsepower
- $20 Target in Noble Capital Markets Report Supported by Live Stream of 1ST Global Super League Kerala Event from AI Powered Sports Leader: $SEGG
- RainbowMe Kids Launches The LoreKeeper's Journal: A Movie-in-a-Box Where Children Become the Hero
- Vesica Health Granted PLA Billing Code for AssureMDx
- Newest Mako Smartrobotics™ System Used for First Time in the State Of Illinois for Total Joint Replacement Surgery
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on ncarol.com
- €6.4 Million in Contracts Across Multiple Countries; Smart City Developer; U.S. Expansion, and Announces Strategic Drone Tech Partnership; $AFFU
- CRYPTOCURRENCY: Lucrumia Exchange Platform Addresses Italian Traders' Growing Demand for Secure Digital Asset Trading
- NIUFO Launches Secure Trading Platform for Italian Market Seeking Stability After 20% User Decline
- OrderDomains.com Empowers Businesses with Premium Domains and Flexible Financing
- Cryptocurrency Trading: AHRFD Enters German Market with Institutional-Grade Infrastructure
- TSWHZC Launches Automated Copy Trading Platform for Brazil's 28 Million Crypto Users
- Keyanb Crypto Exchange Unveils Comprehensive Platform Architecture for Chilean Traders Seeking Lower Fees and Enhanced Security
- Phoenix Advocacy Network Launches to Amplify Survivor Voices and Advance Disability Rights
- Matecrypt Platform Delivers Comprehensive Solution for Argentine Traders with 200+ Cryptocurrencies and 2 Million Orders Per Second Processing
- Wzzph Exchange Expands Brazilian Market Access with Comprehensive Trading Platform
- CCHR: Prescription Psychotropics Fuel America's Addiction and Overdose Crisis
- The Truth Behind Egypt's Stolen Legacy: Livestream
- Digital Pharma Advances 2026: AI and Patient-Centric Strategies Transform Pharma Marketing
- 'ChilCorp – Water' Builds Momentum After Being Named as a Qualified Team in $119M XPRIZE Water Scarcity Global Competition
- Cryptocurrency Exchange AZETHIO Targets US Market Security Concerns with MPC Technology and 15-Minute KYC Verification
- Gospel Sensation "Bible, Faith, Rembrandt aka BFR, They're Taking the World by Storm
- Durex Products Screen Media Suitable for U.S. FAST 41 Critical Minerals Mining Projects
- SQHWYD Launches Cognitive Finance Platform with Intelligent Trading Technology and Unified DeFi Access for Brazilian Market
- Southland Symphony Orchestra Season Opener – A Musical Mosaic
- Iguabit Unveils Comprehensive Platform Strategy for Brazilian Crypto Traders Seeking Regulated Solutions