Trending...
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
Analyst D. Boral Targets NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP at $34 Per Share — Pioneering Breakthroughs in Treatment-Resistant Depression and Chronic Pain
MIAMI - ncarol.com -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical innovator developing breakthrough therapeutics for central nervous system disorders, has emerged as a potential leader in the next generation of psychiatric care. With the global ketamine market projected to expand from $750 million to $3.35 billion by 2034, NRXP is strategically positioned to capture a substantial share through a suite of FDA-designated and investigational treatments aimed at addressing urgent mental health needs.
Revolutionary "ONE-D" Depression Treatment Launches in Florida
NRx recently announced the first-in-Florida launch of its "One Day" (ONE-D) depression treatment, developed in partnership with Ampa Health. The ONE-D protocol uses Ampa's advanced Transcranial Magnetic Stimulation (TMS) device — the first reported technology capable of achieving remission from treatment-resistant depression in a single day.
Unlike conventional 90-day TMS regimens, the ONE-D treatment integrates a single day of precision TMS with D-cycloserine and lisdexamfetamine (both utilized under physician supervision), achieving up to 87% response and 72% remission rates in peer-reviewed nonrandomized studies.
The technology is initially deployed at multiple NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, with expansion to six Florida locations by year-end 2025 under the direction of Dr. Rebecca Cohen, Medical Director of HOPE Clinics.
More on ncarol.com
NRX-101: A Breakthrough Therapy for Suicidal Bipolar Depression
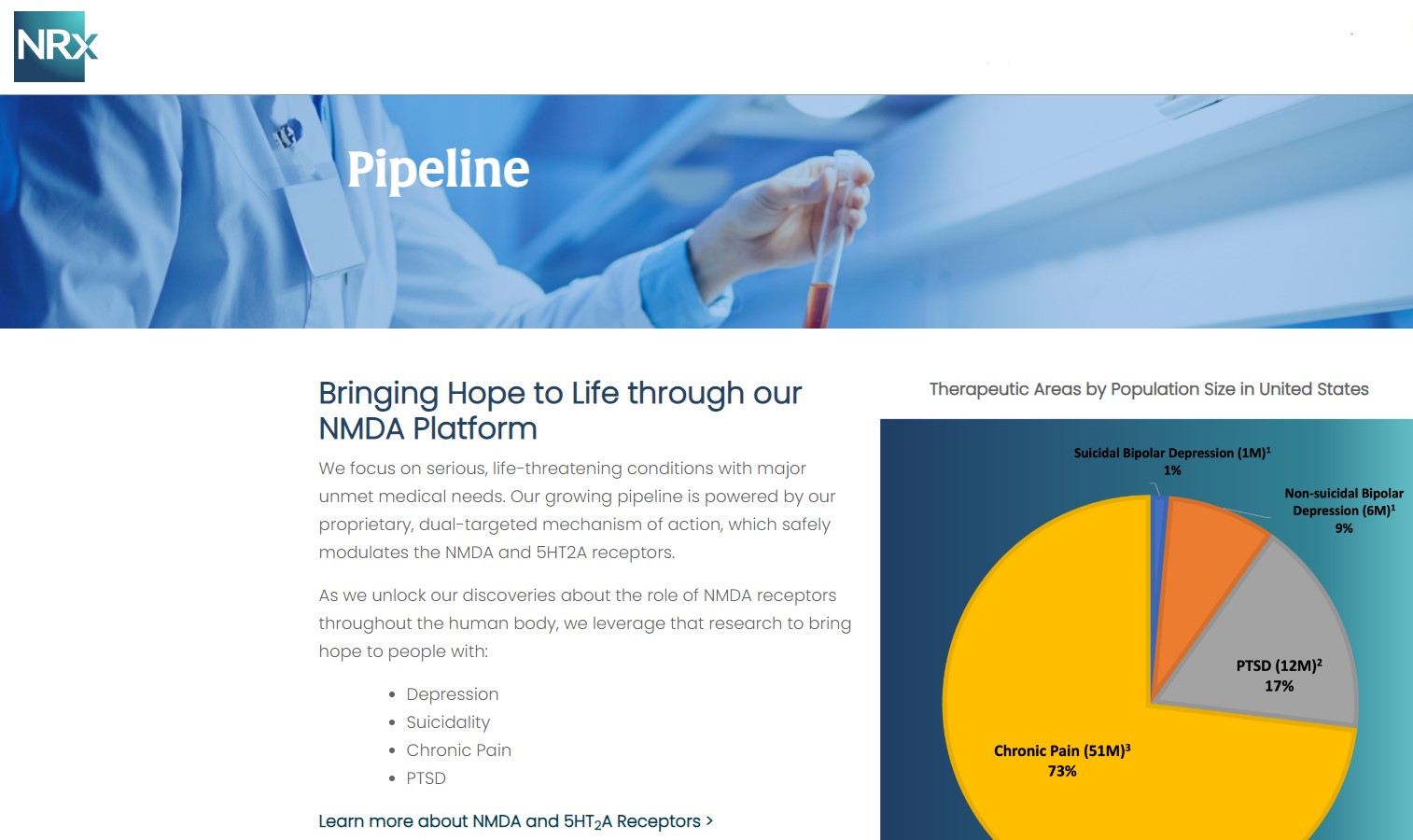
Central to NRx's pipeline is NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. The therapy leverages the company's proprietary NMDA platform and is being developed in partnership with Alvogen Pharmaceuticals.
NRX-101 also holds potential as a non-opioid pain treatment and for other indications such as complicated urinary tract infections (UTIs). With over 13 million Americans seriously considering suicide each year (CDC), NRXP's drug candidates could address one of the nation's most pressing health crises.
KETAFREE™: FDA-Focused Push to Modernize Ketamine Treatment
NRXP recently re-filed its Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free IV ketamine formulation, after securing FDA approval of its Suitability Petition. By eliminating benzethonium chloride (BZT) — a preservative linked to neurotoxicity — KETAFREE™ represents a safer, next-generation alternative to traditional ketamine products.
The company has also petitioned the FDA to formally remove BZT from IV formulations, citing expert toxicological data and the growing demand for preservative-free therapies in modern medicine. Given the ongoing ketamine shortage reported by the American Society of Hospital Pharmacists, NRXP's KETAFREE™ could fill a critical supply gap and strengthen its market positioning.
Strategic Acquisitions and Growth Initiatives
NRXP recently completed the acquisition of Dura Medical, an EBITDA-positive network of interventional psychiatry clinics delivering advanced therapies for depression and PTSD. This acquisition, along with pending deals for Neurospa TMS and Cohen & Associates, expands NRXP's reach to more than eight Florida clinic locations, reinforcing its vertically integrated mental health care model.
More on ncarol.com
To accelerate this rollout, NRXP secured $7.8 million in debt financing from Universal Capital, LLC to fund further HOPE Clinic acquisitions.
Additionally, NRXP has accepted non-binding licensing terms for NRX-100, a drug candidate expected to generate over $300 million in potential milestone payments and tiered double-digit royalties upon commercialization.
Strong Analyst Endorsement
In a recently released report, D. Boral Research issued a Buy rating on NRXP with a $34 price target, citing the company's expanding clinical pipeline, growing footprint in the interventional psychiatry sector, and first-mover advantage in the transformative ONE-D treatment platform.
A Vision for Transforming Mental Health
From its FDA Fast Track designation for IV ketamine (NRX-100) to its breakthrough therapy designation for NRX-101, NRx Pharmaceuticals continues to redefine the boundaries of psychiatric medicine. Its commitment to science-driven innovation — now coupled with real-world clinic deployment through HOPE Clinics — positions NRXP as both a therapeutic pioneer and a potentially lucrative opportunity in the rapidly expanding mental health market.
Company Contact:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
📍 Wilmington, DE
Matthew Duffy, Chief Business Officer
📧 mduffy@nrxpharma.com | ☎️ (484) 254-6134
🌐 www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Revolutionary "ONE-D" Depression Treatment Launches in Florida
NRx recently announced the first-in-Florida launch of its "One Day" (ONE-D) depression treatment, developed in partnership with Ampa Health. The ONE-D protocol uses Ampa's advanced Transcranial Magnetic Stimulation (TMS) device — the first reported technology capable of achieving remission from treatment-resistant depression in a single day.
Unlike conventional 90-day TMS regimens, the ONE-D treatment integrates a single day of precision TMS with D-cycloserine and lisdexamfetamine (both utilized under physician supervision), achieving up to 87% response and 72% remission rates in peer-reviewed nonrandomized studies.
The technology is initially deployed at multiple NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, with expansion to six Florida locations by year-end 2025 under the direction of Dr. Rebecca Cohen, Medical Director of HOPE Clinics.
More on ncarol.com
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
NRX-101: A Breakthrough Therapy for Suicidal Bipolar Depression
Central to NRx's pipeline is NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. The therapy leverages the company's proprietary NMDA platform and is being developed in partnership with Alvogen Pharmaceuticals.
NRX-101 also holds potential as a non-opioid pain treatment and for other indications such as complicated urinary tract infections (UTIs). With over 13 million Americans seriously considering suicide each year (CDC), NRXP's drug candidates could address one of the nation's most pressing health crises.
KETAFREE™: FDA-Focused Push to Modernize Ketamine Treatment
NRXP recently re-filed its Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free IV ketamine formulation, after securing FDA approval of its Suitability Petition. By eliminating benzethonium chloride (BZT) — a preservative linked to neurotoxicity — KETAFREE™ represents a safer, next-generation alternative to traditional ketamine products.
The company has also petitioned the FDA to formally remove BZT from IV formulations, citing expert toxicological data and the growing demand for preservative-free therapies in modern medicine. Given the ongoing ketamine shortage reported by the American Society of Hospital Pharmacists, NRXP's KETAFREE™ could fill a critical supply gap and strengthen its market positioning.
Strategic Acquisitions and Growth Initiatives
NRXP recently completed the acquisition of Dura Medical, an EBITDA-positive network of interventional psychiatry clinics delivering advanced therapies for depression and PTSD. This acquisition, along with pending deals for Neurospa TMS and Cohen & Associates, expands NRXP's reach to more than eight Florida clinic locations, reinforcing its vertically integrated mental health care model.
More on ncarol.com
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- tukr box Ministries Launches Meal-Sharing Kit Partnership With Marry Me Marinara
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
To accelerate this rollout, NRXP secured $7.8 million in debt financing from Universal Capital, LLC to fund further HOPE Clinic acquisitions.
Additionally, NRXP has accepted non-binding licensing terms for NRX-100, a drug candidate expected to generate over $300 million in potential milestone payments and tiered double-digit royalties upon commercialization.
Strong Analyst Endorsement
In a recently released report, D. Boral Research issued a Buy rating on NRXP with a $34 price target, citing the company's expanding clinical pipeline, growing footprint in the interventional psychiatry sector, and first-mover advantage in the transformative ONE-D treatment platform.
A Vision for Transforming Mental Health
From its FDA Fast Track designation for IV ketamine (NRX-100) to its breakthrough therapy designation for NRX-101, NRx Pharmaceuticals continues to redefine the boundaries of psychiatric medicine. Its commitment to science-driven innovation — now coupled with real-world clinic deployment through HOPE Clinics — positions NRXP as both a therapeutic pioneer and a potentially lucrative opportunity in the rapidly expanding mental health market.
Company Contact:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
📍 Wilmington, DE
Matthew Duffy, Chief Business Officer
📧 mduffy@nrxpharma.com | ☎️ (484) 254-6134
🌐 www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on ncarol.com
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Fayetteville Concrete Contractors Serves Cumberland County with Concrete Solutions
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
- 5-Star Duncan Injury Group Expands Personal Injury Representation to Arizona
- NC State and Railinc Partner to Advance Freight Efficiency through Modal Rebalancing Study
- The End of "Influencer" Gambling: Bonusetu Analyzes Finland's Strict New Casino Marketing Laws
- AI-Driven Cybersecurity Leader Gains Industry Recognition, Secures $6M Institutional Investment, Builds Momentum Toward $16M Annual Run-Rate Revenue
- TRIO Heating, Air & Plumbing Now Ranks #1 in San Jose
- Milwaukee Job Corps Center Hosts Alumni Day, Calls Alumni to Action on Open Enrollment Campaign
- Golden Paper Identifies Global Growth in Packaging Papers and Upgrades Its High-End Production Capacity
- Champagne, Caviar Bumps & Pole Performances — Welcome the New Year Early with HandPicked Social Club
- A New Soul Album: Heart Of Kwanzaa, 7-Day Celebration
- Allegiant Management Group Named 2025 Market Leader in Orlando by PropertyManagement.com
- Local upholstery business expands services to meet growing demand in Shelby and Cleveland County
- NAFMNP Awarded USDA Cooperative Agreement to Continue MarketLink Program Under FFAB
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge