Trending...
- AltQuick.com Announces Continued Support for Bitcoin Testnet 3 Trading Amid Testnet 4 Launch
- Peak 10 Marketing Expands Capabilities and Opens Doors to New Clients
- Charlotte Nurse-Leader Dr. Andrea "Angel" Taylor Joins the Dear Black Woman Anthology
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Continues Expansion with Completion of Dura Medical Acquisition in Network of Interventional Psychiatry Clinics
MIAMI - ncarol.com -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage with Buy and $40 Price Target.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
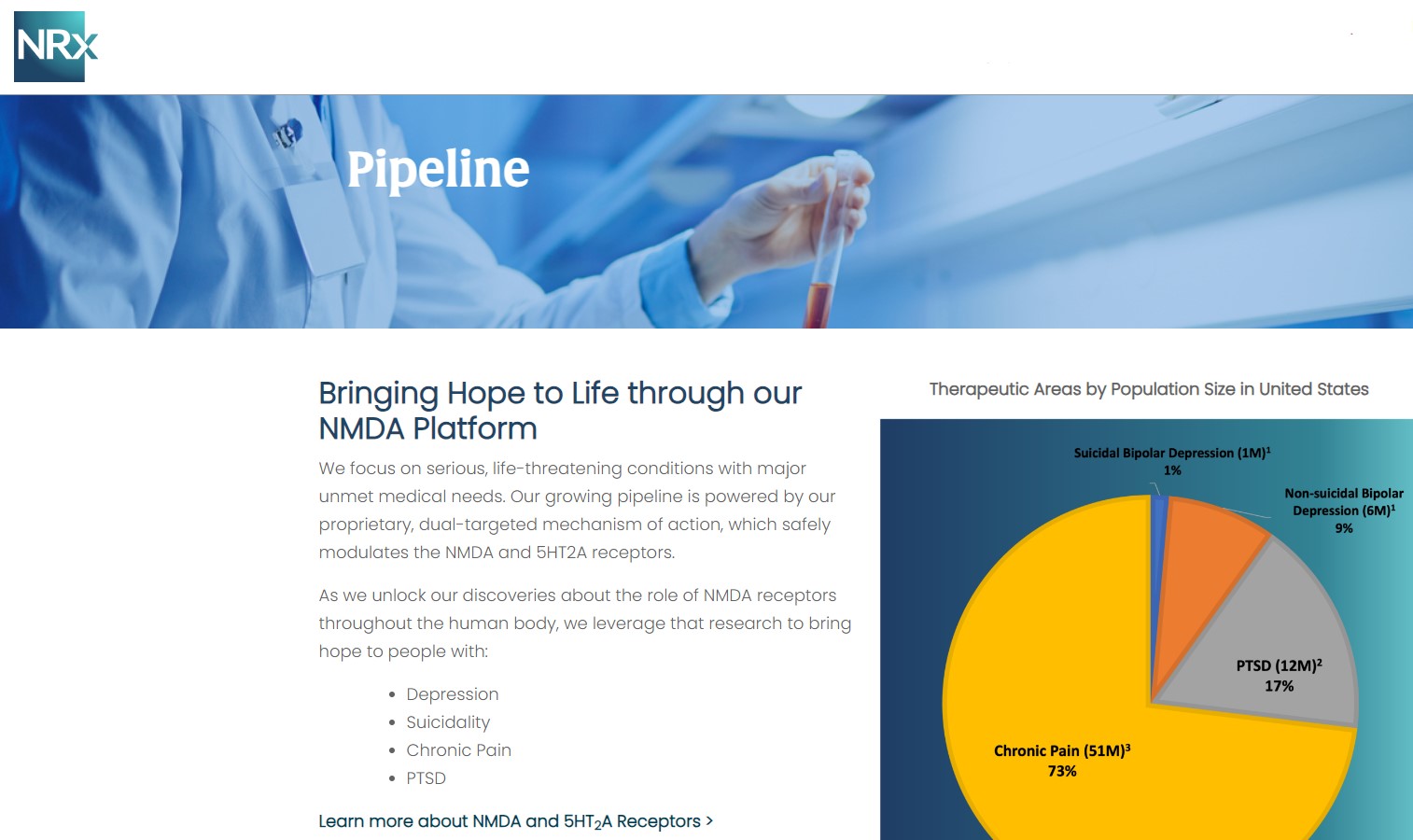
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
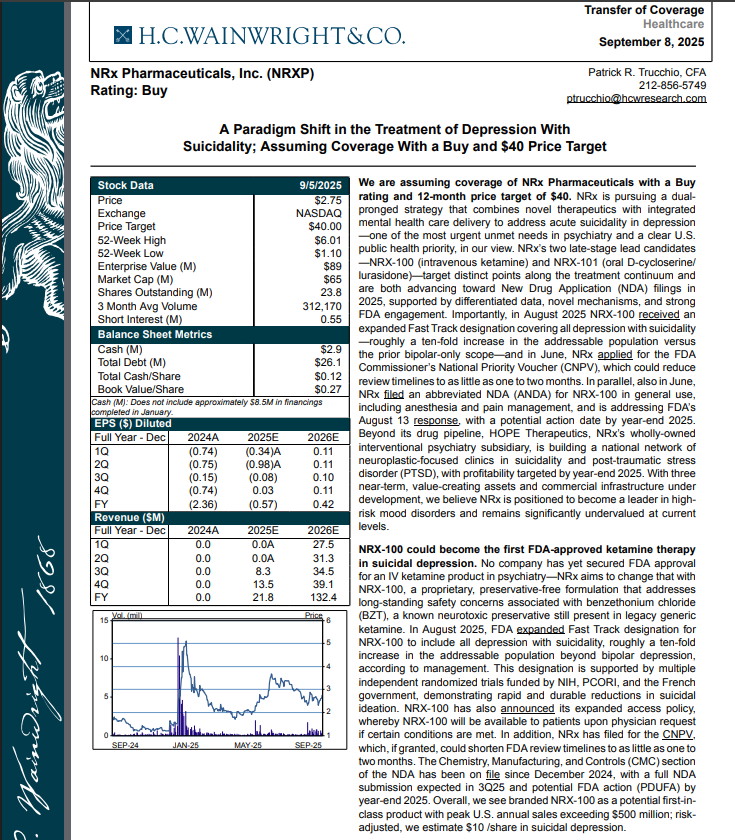
On September 8th H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on ncarol.com
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Expanded Access Policy for NRX-100 (preservative-free ketamine)
On August 27th NRXP announced its expanded access policy for NRX-100 (preservative-free ketamine) based on grant of Fast Track designation for NRX-100 in the treatment of suicidal ideation in patients with depression, including bipolar depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet need, based on an assessment of the preliminary data contained in the Fast Track designation request. Accordingly, NRXP NRX-100 is available for expanded access to eligible patients.
Second Quarter 2025 Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
More on ncarol.com
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression With Suicidality; Assuming Coverage with Buy and $40 Price Target.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
On September 8th H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on ncarol.com
- New Slotozilla Project Explores What Happens When the World Goes Silent
- The Two Faces of Charles D. Braun: How the Novel, Posthumously Yours, Came to Life
- Counseling Center of New Smyrna Beach Expands Affordable Mental Health Services for Volusia County
- Marty the Martian Farmer: A Retro Sci-Fi Comedy with a Cosmic Twist
- Athena Forge (ATFG) Introduces Advanced Token for Technology-Driven Financial Ecosystem
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Expanded Access Policy for NRX-100 (preservative-free ketamine)
On August 27th NRXP announced its expanded access policy for NRX-100 (preservative-free ketamine) based on grant of Fast Track designation for NRX-100 in the treatment of suicidal ideation in patients with depression, including bipolar depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet need, based on an assessment of the preliminary data contained in the Fast Track designation request. Accordingly, NRXP NRX-100 is available for expanded access to eligible patients.
Second Quarter 2025 Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
More on ncarol.com
- Albuquerque's Z-CoiL Footwear Brings All-American Family Business Story to Shark Tank Season Premiere
- NoviSign Sponsoring VARTECH 2025 - the B2B IT channel's #1 event
- Unicorp and BH Group Select Chasing Creative—Palm Coast Agency—to Lead Growth Marketing for The Ritz-Carlton Residences, Hammock Dunes
- Breaking: 50+ runners from 20+ states relay custom 9/11 flag 485 miles from Shanksville through DC to Ground Zero for memorial remembrance run
- SecureMaine 2025 is this October 8th in Portland, Maine
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on ncarol.com
- Nashville International Chopin Piano Competition Partners with Crimson Global Academy to Support Excellence in Education
- Entrinsicon 2025 Returns to Raleigh, Showcases AI and Data Innovation
- AHRFD Releases Market Analysis: Cryptocurrency Market's Institutional Transformation Accelerating
- Ubleu Crypto Group Analyzes European Digital Asset Market Opportunities Amid Regulatory Evolution
- NIUFO Examines European MiCA Regulation's Impact on Digital Asset Trading Markets
- Wzzph Analyzes Crypto Market Maturation as Institutional Capital Drives $50B ETF Inflows
- GXCYPX Analyzes South America's Emerging Digital Asset Market Dynamics
- Keyanb Crypto Exchange Positions for Latin America's $600 Billion Remittance Opportunity Amid Global Regulatory Shifts
- NAQSN Analysis: $2.75 Trillion Digital Asset Market Demands Unified Infrastructure
- Trinity Accounting Practice Celebrates 22 Years Serving Beverly Hills Businesses
- YuanziCoin Unveils Revolutionary Shariah-Compliant Blockchain Architecture for 1.8 Billion Muslims Worldwide
- The Hidden Crisis No One is Talking About
- AGEIMMUNE Launches Brilliant D3 + Magnesium & K2: A Doctor-Formulated Supplement for Bone, Immune & Heart Health Support
- YMCA of the Jersey Shore Helps Residents Take Control of Health
- Z-CoiL Footwear, Albuquerque's Original Spring Shoe, Steps Into ABC's Shark Tank Season Premiere
- How LIB's Temperature & Humidity Chamber & Walk-in Chamber Warranty Delivered Real Uptime
- Peak 10 Marketing Expands Capabilities and Opens Doors to New Clients
- Charlotte Nurse-Leader Dr. Andrea "Angel" Taylor Joins the Dear Black Woman Anthology
- AltQuick.com Announces Continued Support for Bitcoin Testnet 3 Trading Amid Testnet 4 Launch
- Platinum Plumbing Expands Services, Rebrands as Platinum Plumbing & Water Well Services